CP Introduction Video
Created as an internal video to train employees, this cathodic protection video is great for anyone that wants to understand the basics of CP. The summary below outlines the topics discussed and their corresponding times in this “CP 101” video.
CHAPTER 1: Introduction to Cathodic Protection
(0:36, 7:07) Cathodic Protection (CP) is an electrochemical process where DC current is applied to a metal to slow or stop corrosion currents. When properly applied, CP stops the corrosion reaction from occurring.
What is Corrosion? (1:32)
The processing of metals into usable materials takes a lot of energy. Corrosion is a reaction where refined metals release that energy and return to their natural state.
How does CP prevent corrosion? (2:52)
Cathodic protection stops the energy from leaving the metal and prevents corrosion by applying current to the surface.
The Corrosion Reaction (3:00)
The corrosion reaction is an oxidation reduction reaction – a chemical reaction where the metal reacts with the environment to reduce it to an oxide form. Corrosion occurs where current leaves the structure.
Corrosion Cell Elements (4:52)
- Anode*
- Cathode*
- Metallic Path*
- Electrolyte
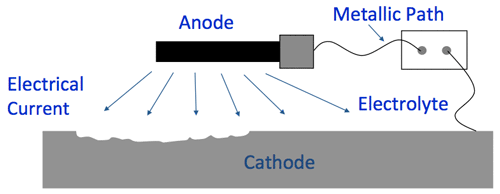
*These are inherent in a metallic structure. Without an electrolyte such as water, there is no corrosion.
(7:07) Cathodic protection works by placing an anode or anodes (external devices) in the electrolyte to create a circuit where current flows from the anode, through the electrolyte to the surface of the structure. Corrosion moves to the anode to stop further corrosion from occurring.
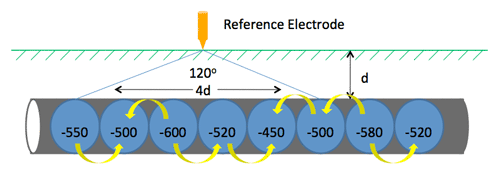
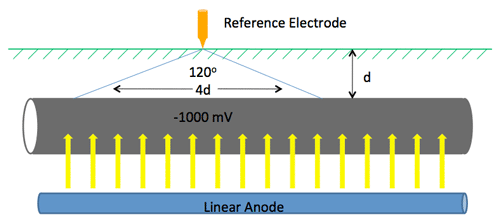
Pipeline CP Example (13:00)
On an unprotected pipeline, there are naturally occurring potential variations. Wherever you go from a minor positive to a minor negative, there is a current flow where galvanic corrosion will occur. (13:45) If you put CP on that pipeline, for example MATCOR’s linear anode that runs parallel to the pipeline, current is discharged off of the anode and onto the pipeline, preventing corrosion.
![]()
Corrosion Rate vs Current (15:35)
The corrosion rate drops as we apply CP. The corrosion rate goes to nearly zero and the structure is protected against corrosion. For some metals, if you apply too much current, you can cause damage to the metal.
CP Criteria (16:41)
NACE has defined 3 criteria in SP0169-2013, “Control of External Corrosion on Underground or Submerged Metallic Piping Systems.”
- 850 mV ON – Structure potential with CP applied to be less than -850mV after accounting for IR losses
- -850 mV OFF – A negative polarized potential of less than -850V the moment CP is turned off (basically the same as 850 mV ON)
- 100 mV polarization shift from native state to CP applied
Can There Be Too Much CP?
Too much current can damage some metals such as stainless steel and titanium. There is also a practical aspect of applying too much current.
CHAPTER 2: Components of a Cathodic Protection System (20:48)
- Structure being protected
- Electrolyte that current can flow through
- Anode system delivering current
- Cabling connecting structures and anode system
- Provisions for testing
(24:00) In the presentation, our expert describes a very common application of CP for a seawall. These structures are difficult to repair if they start to fall apart from corrosion. A deep well anode system can protect the entire structure.
Two Types of Anodes Systems (26:40)
The presentation goes into the two types of cathodic protection anodes, the history, various configurations, and the advantages and disadvantages of each.
Galvanic vs. Impressed Current Anodes
| Advantages | Disadvantages | |
|---|---|---|
| Galvanic Anodes (Sacrificial Anodes) (29:35) |
|
|
| Impressed Current Anodes (35:50) |
Advantages apply to MMO impressed current anodes |
|
Mixed Metal Oxide (MMO) Anodes (38:31)
MMO anodes have become the anode of choice for most applications. Mixed metal oxide anodes consist of a coating over a titanium substrate. They have a negligible consumption rate and are available in a wide range of configurations including:
- Spheres
- Ribbon
- Wire
- Rods
- Tubes
- Plates
- Mesh
Additional Components in a CP System (40:38)
- Anode Backfill Material (40:38)
- Cathodic Protection Rectifier (41:20)
- Test Stations (42:23)
- Reference Electrode (43:10)
CHAPTER 3: CP Design Steps (43:58)
- Evaluate the Structure (44:26)
- Evaluate the Environment (44:03)
Question: How does temperature affect corrosion?
Answer: As a general rule of thumb, the corrosion rate doubles for every 10℃ increase in temperature. (47:42) - Anode Placement and Constraints (48:57)
- Determine Current Required (50:00)
A sample calculation for the CP current required for an above ground storage tank (AST) is provided in the video at 51:35 - Determine Current Density Required (52:44)
- Anode Selection (54:17)
Anode system resistance is a crucial consideration when selecting anode type and configuration. (55:29) - Calculate Design Life (57:34)
Cathodic Protection System Design is both quantitative and qualitative (59:00)
CHAPTER 4: Anode Configurations (59:51)
There are 3 basic anode configurations:
- Remote Anodes
- Discrete Anodes
- Linear Anodes
Ground Bed Definition: Ground bed is a generic term for anode installations where one or more anodes are installed in the ground. (1:00:30)
There are two categories of ground beds for installing anodes:
- Shallow Ground Beds – where the anodes are located near the surface
- Deep Well Ground Beds – where the anodes are located in drilled wells, typically at depths exceeding 100 ft (33m). A typical deep well anode configurations is described at 1:02:21 in the presentation. Congested plant environments, sheet pile walls and long distance pipelines are typical applications for deep well CP systems.
Remote Anode Systems (1:01:00)
Remote ground bed anode systems are used to project current over a wide area and placed a distance away from the structure being protected.
- Electrically remote from the structure
- Can be deep anode ground beds or remote horizontal ground beds (1:05:01).
Discrete Anode Systems (1:06:30)
Discrete anode systems are individual anodes located at a short distance from the structure being protected.
- 5-10 feet away from structure
- 5-10 feet depth
- Can be Galvanic or impressed current
Linear Anode Systems (1:10:19)
Linear anodes systems are installed parallel to the structure being protected. They are frequently used for new construction pipeline and construction plant applications.
- Low power system
- Easy to install
- Very reliable
- Long life: 50-100 years
Learn More
Cathodic Protection Products & Materials
Cathodic Protection Services
To get in touch with our team of cathodic protection experts for more information, to ask a question or get a quote, please click below. We will respond by phone or email within 24 hours. For immediate assistance, please call +1-215-348-2974.
Contact a Corrosion Expert





